Complications and Toxicities of Implantable Biomaterials Used in Facial Reconstructive and Aesthetic Surgery: A Comprehensive Review of the Literature
The use of implantable biomaterials has become an integral part of aesthetic and reconstructive surgery of the face. Metals are used for fracture fixation devices, whereas polymers are used primarily for bone or soft-tissue substitution. This review of the scientific literature examines the risks and complications of these materials. First, we present an overview of commonly used materials. Second, we address general considerations of toxicity relevant to all biomaterials. Third, we present data from a large number of clinical series on the incidence of complications for individual materials used in specific applications.
IMPLANT MATERIALS
The demands placed on alloplastic implants are high. Ideally, we strive to use a material that is cost effective, but not toxic, antigenic, or carcinogenic. The material should be inert in body fluids, easily shaped at the operating table, and maintain its desired form and consistency in situ. Most importantly, the ideal implant would be permanently accepted. A variety of materials have been used for implants in facial aesthetic and reconstructive surgery, some coming closer than others in approaching the qualities of an ideal implant material. No one material, however, is universally successful.
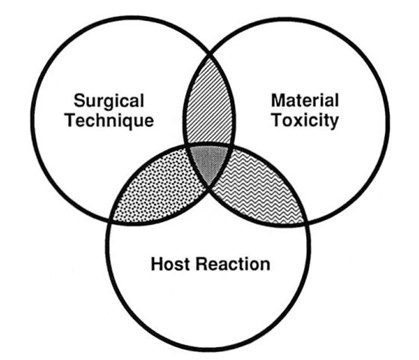
Biocompatibility, or the interaction between host and implant material, is influenced by many factors (Fig. 1). The physical characteristics of the implant material, including firmness and surface characteristics, play an important role. Moreover, the implant material itself or its breakdown products could have toxic potential. The host reaction to the implant can vary from patient to patient, and may be affected by any number of variables that influence wound healing, such as systemic illness or medications. Surgical technique, including preparation of the material, size of the device, and method of mechanical fixation, can have a tremendous effect on the fate of an implant. All of these elements must be considered when using alloplastic materials.
Metals
The three types of metal currently used for fixation devices are stainless steel, cobalt- chromium alloy (Vitallium), and titanium (either pure Ti or as an alloy with aluminum and vanadium). Titanium has become a popular metal for craniofacial use because of its high strength and reduced artifact on CT and MR imaging studies.1,2
Polymers
Polymers are long molecules composed of repeating subunits. Structure of the subunit, chain length, and cross-links all determine physical properties of the material. The subunit structures of common polymers are shown in Figure 2.
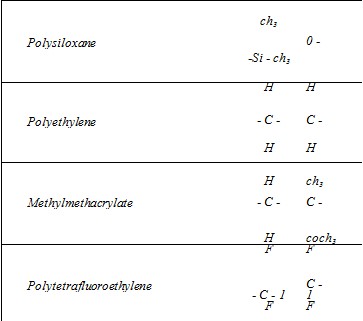
Polysiloxane (silicone). Although bearing the chemical name polysiloxane, this ubiquitous material is commonly referred to as silicone. Probably the most familiar implant material, silicone can be manufactured with consistencies ranging from liquid to hard solid. It also comes in a room temperature vulcanized (RTV) form. In contradistinction to the standard heat temperature vulcanized (HTV) form, RTV silicone is mixed with a hardening agent and custom molded by the surgeon either before implantation or in situ. The natural host response to this smooth surfaced material is fibrous encapsulation or, if soft-tissue cover is thin or under tension, extrusion of the implant.3,4 The manufacturing process plays an important role in the purity and integrity of this material.
Polyethylene. This biomaterial has a simple carbon chain structure. It serves as the reference standard for an inert substance in assays of tissue reaction to biomaterials.5,6 Like silicone, it can be produced in a variety’ of consistencies from grease to hard solid. A porous form of polyethylene such as Medpor (Porex, Fairburn, Ga.) is most commonly used. The porous structure allows ingrowth of both soft tissue and bone,7 which may make the implant very difficult to remove.
Polytetrafluoroethylene. Best recognized as Gore-Tex (W. L. Gore, Flagstaff, Ariz.), this material is produced in pliable sheets that can be easily cut and shaped. Small interstices allow for some tissue ingrowth.
Proplast.
Proplast (formerly by Vitek, Houston, Texas) is a composite material consisting of polytetrafluoroethylene combined with either carbon fibers (Proplast I) or aluminum oxide (Proplast II). It is a porous implant material that is no longer manufactured in the United States.
Methylmethacrylate. Commonly used as a bone cement, this material is formed by mixing powdered polymer with liquid monomer. The resultant chemical reaction is highly exothermic, and toxicity has been associated with the free monomer. Hardened methylmethacrylate can be brittle and prone to fracture.
HTR. HTR (Biomet, Inc., Warsaw, Ind.) derives its name from the acronym for hard tissue replacement. It is a porous composite of polymethylmethacrylate and polyhydroxyethyl methacrylate. A calcium hydroxide coating imparts a negative surface charge to encourage bony ingrowth.8
Polypropylene. This polymer is used in the manufacture of nonabsorbable sutures and most types of surgical mesh. Although not specifically marketed as a tissue augmentation device, surgical mesh has been used for this purpose in a number of patients.
Polyamide. Nylon is the general name used to describe a group of polymerized amides. A smooth surfaced nylon plate is produced under such trade names as Suprafoil (S. Jackson, Alexandria, Va.). Reported high extrusion rates may be due to inherent rigidity of the material.
Bioplastique. Bioplastique is an experimental injectable implant device composed of silicone microbeads in a bioexcretable gel vehicle. Its use has recently moved to the clinical arena.9Â Resorbable Plates and Screws. Resorbable fixation devices for craniofacial surgery are primarily composed of polylactic acid, or a copolymer of polylactic acid and polyglycolic acid. Polydioxanone is also used. These materials degrade by hydrolysis.10
Ceramics
Ceramic materials are usually heated to high temperatures (sintered) to fuse their crystalline components. One ceramic commonly used for implants in craniofacial surgery is hydroxyapatite. Hydroxyapatite is a calcium phosphate salt found as a major component of bone matrix. Dense hydroxyapatite can be produced synthetically. Porous hydroxyapatite can be entirely synthetic, or formed by chemically converting the naturally porous calcium carbonate skeleton of marine coral. Porous hydroxyapatite is too brittle to bear a load, but will allow bony ingrowth when used as an onlay or inter- positional implant. Absorption of hydroxyapatite is minimal.11
GENERAL CONSIDERATIONS OF IMPLANT TOXICITY
Carcinogenicity
The ability of implantable devices to induce malignancy locally or systemically becomes a question of concern, especially when they are placed in young patients with a long life expectancy. It is estimated that 88,200 hip replacements were performed in 1975,12 and that 11 million people currently carry implanted metallic fixation devices.13 With this large number of implants in place and a 20-year latency period passed, one might expect a gradually increasing number of implant related cancers to be emerging if the risk of malignancy was significant. Fortunately, clinical observations support the hypothesis that the risk of cancer with metal implant devices is extremely low. Only 20 cases of malignancy in association with metal fixation devices have been reported in the literature14-32 with latencies ranging between 1 and 30 years. Only one of these cases occurred above the neck,30 and it differed from other implant related tumors because the histology showed squamous cell carcinoma rather than sarcoma. This particular tumor occurred at the point where a mandibular staple bone plate contacted the gingiva.
Although the risk of local cancer with metal implants appears to be very low, there is still a question of increased risk for cancer systemically. Gillespie et al.33 found a significant in-crease in the risk of lymphatic and hemopoi¬etic tumors in 1358 patients after total hip replacement. The authors of that study, though, are quick to caution against drawing causal relationships because many other factors associated with arthritic disease may have contributed to the increased cancer rate.
Cancers in association with polymer implants also appear to be rare. Five cases of sarcoma associated with Dacron vascular pros- theses (latency 6 months to 5 years) appear in the literature.34-38 No cases of cancer have been reported in association with a polymer craniofacial implant. The low risk of malignancy with polymer implants is further supported by studies examining the relationship between breast implants and cancer. In one series, Deapen et al.39,40 studied the incidence of breast cancer in 3112 patients in the Los Angeles area after aesthetic breast augmentation with average follow-up at both 6.2 and 10.6 years after implantation. In another series, Berkel et al.41 studied a cohort of 11,676 women with an average follow-up of 10.2 years. Bryant and Brasher42 published a reanalysis of Berkel’s data confirming the conclusions. Both of these cohort studies showed no increased risk of breast cancer after augmentation mammoplasty.
Systemic Disease
In addition to the question of malignancy associated with implant devices, one must consider the possibility that injury may occur to one or more organ systems as a result of the material.
Corrosion of metal implants leads to the release of metal ions, raising questions about the risk of systemic toxicity from these degradation products. In humans, accumulation of metal has been found adjacent to maxillofacial fixation plates43,44 and in a submandibular lymph node after mandibular plating.45 The corrosion process can result in a local fibroblastic tissue reaction22,46 which has been implicated in implant failure in maxillofacial plates47 and long- bone fixation devices.48 Moreover, postmortem studies of humans with stainless steel and cobalt-chromium long-bone implants demonstrated elevated levels of these metals in local and distant lymph nodes, bone marrow, liver, and spleen.49 Despite the documented spread of degradation products from metal implants and the fact that metals such as titanium have been linked to necrotizing processes after industrial exposure,50 no direct associations between implant corrosion products and systemic disease have been drawn to date.
Recent controversy over a possible link between silicone breast implants and connective tissue diseases has prompted the publication of two well-controlled studies. In the first study, Gabriel et al.51 investigated the risk of connective tissue disease in 749 women with breast implants followed for an average of 7.8 years. In the second study, Sanchez-Guerrero et al.52 studied 1183 members of the Nurses Health Study Cohort with breast implants followed an average of 9.9 years. Neither study found an increased risk for connective tissue diseases after silicone breast augmentation.
Systemic toxicity to silicone has been documented after injection of large volumes of liquid silicone for breast augmentation. Ellenbo- gen et al.53 reported two cases of silicone induced granulomatous hepatitis confirmed by liver biopsy and one case of lethal pulmonary edema after inadvertent intravascular injection of liquid silicone. Systemic toxicity has also been reported with the use of methylmethacrylate. During the insertion of hip prostheses with freshly mixed methylmethacrylate into the femur, patients have suffered acute hypotension that can progress to cardiovascular collapse and death.54-56 The free monomer form of methylmethacrylate has been shown experimentally to have direct vasodilatory effects. Presumably, the monomer leaches out of the cement and into the exposed medullary vasculature before the compound is fully cured.57 The large volume of methylmethacrylate used to cement a hip prosthesis, combined with a large intramedullary surface area, likely provides the best setting for this type of reaction. No episodes of cardiovascular depression have been reported with the use of methylmethacrylate in craniofacial surgery.
HYPERSENSITIVITY
An uncommon reaction to metal implants is a type IV (cell mediated) hypersensitivity reaction resulting in sequelae such as pain, nonunion of bone, and overlying dermatitis. This has been reported with Vitallium58,59 and stainless steel,60-62 and suggested for titanium.63-65 In maxillofacial use, hypersensitivity reactions have been noted with the use of stainless steel wire66-67 and plates.68 Sensitivity is often confirmed by patch testing, and removal of the implant resulted in resolution of symptoms in all reports.
True hypersensitivity reactions to prefabricated polymer implants are almost unheard of. The free monomer of methylmethacrylate, however, has been noted to cause asthmatic reactions in operating room staff during surgical procedures. A high concentration of vaporized monomer during the curing process can affect sensitized individuals, especially if the room is not properly ventilated.69
APPROACHING SPECIFIC IMPLANT COMPLICATIONS
We reviewed nearly 200 clinical studies reporting series of patients with implantable biomaterials in the face. Studies that failed to provide information concerning both the total number of patients and the specific number of complications encountered were excluded. Complication rates were then extracted from the remaining papers and entered into a computer database. The data were tabulated and weighted averages by sample size were calculated for different complications of materials used in various applications. Wherever possible, complication rates from studies describing use of a single material at various sites were separated by implant site. Case reports describing less common complications were collected for discussion, but not included in the database or in data tabulations.
Only complications related to the implant material itself were included in the data analysis. Poor outcomes relating closely to surgical technique were excluded from the list of implant related complications. Examples are hematoma, nerve injury, overcorrection of enophthalmos, and placement of improperly sized malar implants. Similarly, patient requests for implant removal because of dissatisfaction or fear of harboring foreign material were also excluded.
The list of designated implant related complications used for data analysis included: infection, fistula, exposure, extrusion, displacement, implant fracture, seroma, persistent edema, prominence, pain, inflammatory reaction, hardware loosening, hypersensitivity, and neoplasm. The last two items on this list occur with sufficient rarity as to not play a significant role in analysis of data from these clinical studies. Moreover, certain complications arising by a similar process were grouped together for simplicity; these include infection/fistula and exposure/extrusion. When reporting on complication rates in this review, we sometimes refer to a study as having no implant related complications (IRC). This does not indicate that the series was free of all complications, but that no complications were reported that matched those on our designated list.
It is difficult to attribute many of the complications listed above solely to the implant material itself. As mentioned earlier, there is much overlap between surgical technique, host response, and potential toxicity of the implant. For example, widely differing perioperative antibiotic regimens combined with varying attention to aseptic technique can influence infection rates for a given material and site. The incidence of displacement of an implant can be influenced by variation in the method of mechanical fixation. One can also argue that pain and prominence are often due to technical factors. Nevertheless, we have attempted to exclude those complications obviously due to surgical technique and include those for which the characteristics of the material could play an important role.
INCIDENCE OF IMPLANT RELATED COMPLICATIONS
Metal Fixation Devices
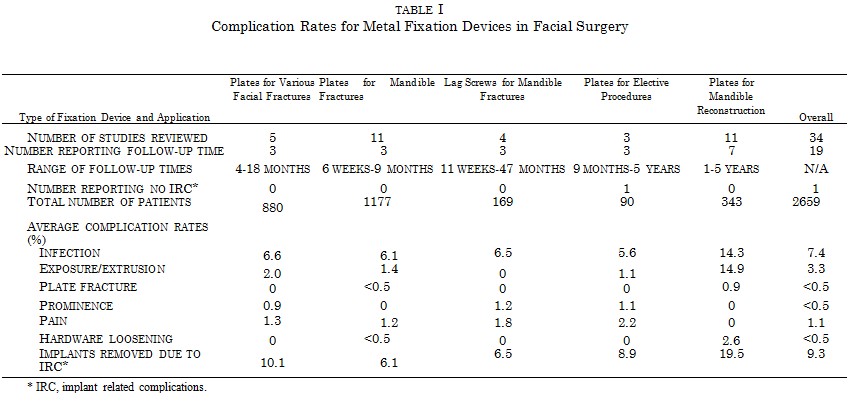
Avast experience with the use of metal plates in craniofacial surgery has been published. Five studies, comprising 880 patients, examined the use of metal plates and screws for facial fractures at various sites.70-74 The average incidence of infection was 6.6 percent, with 2 percent of the implants becoming exposed (Table I). Prominence was reported in 0.9 percent of patients and pain in 1.3 percent. Overall, 10.1 percent of plates were removed for implant related complications. A slightly lower incidence of infection was seen with plates used for elective craniofacial procedures, although the numbers of patients in these series were smaller.70,75,76 Plates used specifically to treat mandible fractures77-89 had a similar incidence of infection (6.1 percent), but prominence was not reported. Plates used to fix mandible fractures are placed under load, and as a result a small incidence of plate fracture (<0.5 percent) and hardware loosening (<0.5 percent) is reported. These latter two complications tended not to occur when lag screws were used to fix mandible fractures.90-93 The highest complication rates with metal plates were observed with mandibular reconstruction after tumor resection, where plates were applied in patients undergoing chemotherapy and radiation treatment.94-104 Infection rates and plate exposure were 14.3 percent and 14.9 percent, respectively. Overall, metal fixation devices had a 7.4 percent incidence of infection and 3.3 percent rate of exposure in 2659 patients. Five studies, encompassing 513 patients,78,82,85,86,105 attempted to compare complication rates of intraosseous wire fixation with plate fixation. An average infection rate of 10 percent was noted with wires. However, these specific studies also reported a much higher average infection rate (14 percent) with plates than most others.
Resorbable Fixation Devices
The use of resorbable plates and screws in craniofacial surgery is a fairly new development and could be especially beneficial in infant patients, where intracranial plate translocation is known to occur. The number of patients with resorbable fixation devices studied is still small, making it impractical to average the complication rates in the same manner as with studies assessing other materials. The results of two studies serve to exemplify problems of prolonged biodegradation that still need to be resolved with this type of implant. Gerlach106 used poly-L-lactide plates and screws to repair zygomatic fractures in 15 patients. Although all fractures and wounds healed uneventfully, the plates were still palpable through the skin after 2 years. At 30 months, two patients developed a noninfectious inflammatory reaction at the operative site. Poly-L-lactide could be aspirated from the area of inflammation. Bos et al.107 used poly-L-lactide plates and screws on 10 patients with zygomatic fractures. Long-term follow-up reported by Bergsma et al.108,109 showed that ail patients experienced acceptable wound and fracture healing. However, all patients in this series developed a sterile foreign body reaction at the repair site at approximately 3 years postoperatively. Fragments of poly-L- lactide were found during wound exploration as long as 5.7 years after the initial repair. Clinical trials with new copolymer plates are being conducted in the United States, but long-term data are not yet available.
Studies Assessing Polymeric and Ceramic Implants at Mixed Sites
Polymers have been used as an implant material in plastic surgery for many decades. One of the longest retrospectives on the use of polyethylene is a 32-year experience published by Rubin.110 He reported an 89 percent success rate for mixed implant sites including malar, nose, mandible, skull, and orbital floor. This study attests to the long-term stability of implantable devices. His custom-made dense polyethylene implants have no modern commercial equivalent, however, and data from this work have not been tabulated with results from other studies using standard porous polyethylene devices.
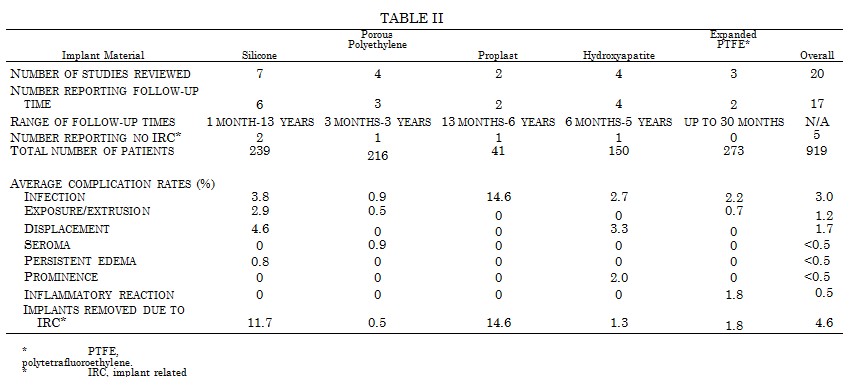
Silicone has been a widely used implant material. Seven studies, describing 239 patients,111-117 reveal an infection rate of 3.8 percent and an exposure/extrusion rate of 2.9 percent for mixed facial implant sites (Table II). Porous polyethylene has enjoyed a much lower infection rate (0.9 percent) and almost no displacement.118-121 Proplast122,123 implants used in mixed sites have a higher infection rate (14.6 percent), but a low number of patients41 are included in the two series reviewed. Hy-droxyapatite124-127 has a favorable infection rate of 2.7 percent, but also carries a 2 percent incidence of prominence. Expanded polytetrafluoroethylene,128-130 when used primarily for subcutaneous tissue augmentation, has a fairly low infection rate of 2.2 percent. Overall, the infection rate for polymeric and ceramic implants at mixed sites is 3.0 percent in 939 patients reviewed.
Complication Rates for Chin Implants
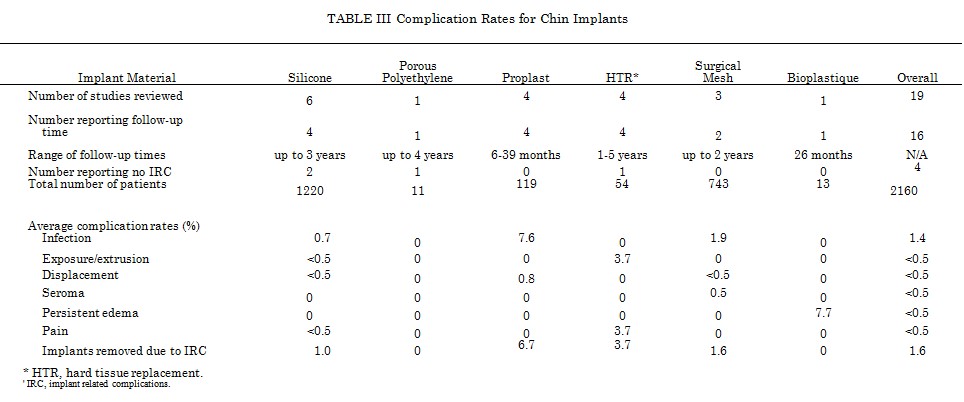
A number of studies report the incidence of complications for different materials used as chin implants 115,123 131-147 (Table III). Silicone chin implants have an average infection rate of 0.7 percent in 1260 patients. Exposure occurs rarely (Fig. 3). Surgical mesh has been a popular material for chin implants, but appears to have had a higher rate of infection (1.9 percent) than silicone in a total of 743 patients. Proplast demonstrates the highest infection rate in this application at 7.6 percent. Porous polyethylene was found to have no implant related complications in a very small series of 11 patients. The overall infection rate for chin implants was 1.4 percent in 2160 patients, and the rate of displacement <0.5 percent. One important feature of implant complications is their potential to occur late in the postoperative period. Loss of a chin implant to infection has occurred 3 years,148 4 years,149 10 years,150 and 47 years 151 after insertion. Late implant infections are usually secondary to bacterial seeding from another active source such as a dental infection.
Bone resorption under chin implants has been an issue of concern since the late 1960s, when it was discovered incidentally on radiographs.152 Further study of this phenomenon revealed that up to several millimeters of bone resorption was likely to occur with a majority of subperiosteal chin implants, but loss of soft- tissue projection or pain did not accompany these bony changes.136,103,154
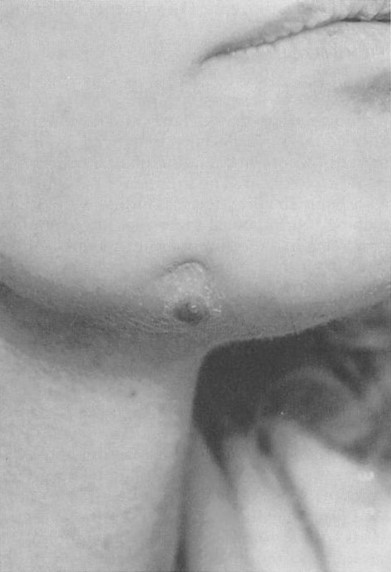
Bone resorption was less likely to occur when the implant was placed in the supraperiosteal position135,154 or over the hard bone of the lower mandible.154
Complication Rates for Malar Implants
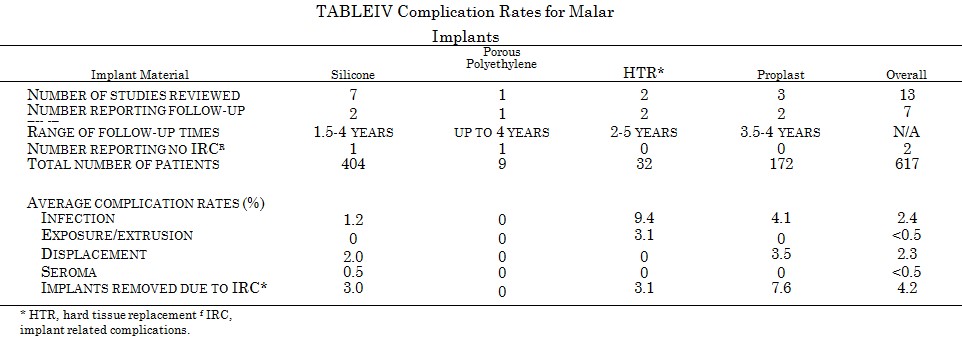
Table IV shows complication rates for silicone,155-161 Proplast,137,162,163 HTR,139,140 and polyethylene143 malar implants. Clinical experience has been most extensive with silicone, where rates of infection and displacement average 1.2 and 2.0 percent, respectively. The overall infection rate for 617 patients with malar implants is 2.4 percent, and the incidence of displacement 2.3 percent. Displacement in this anatomic site can be highly influenced by implant shape and method of fixation.
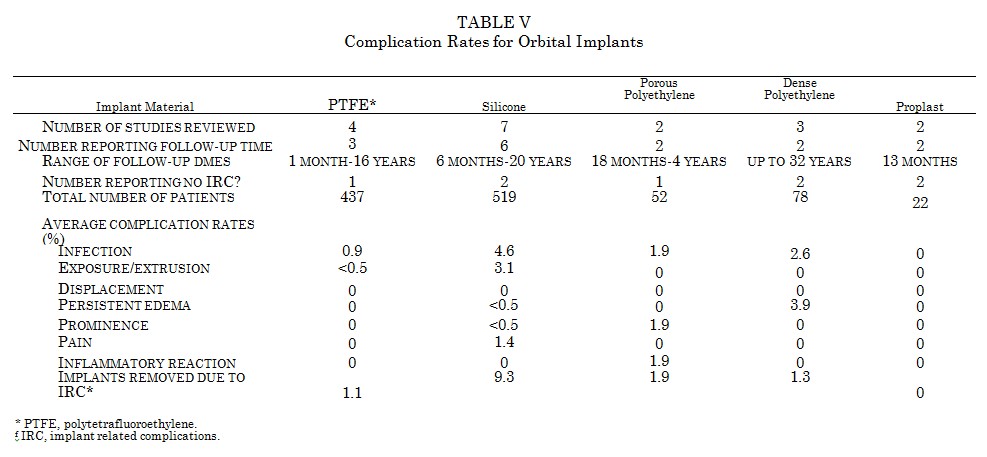
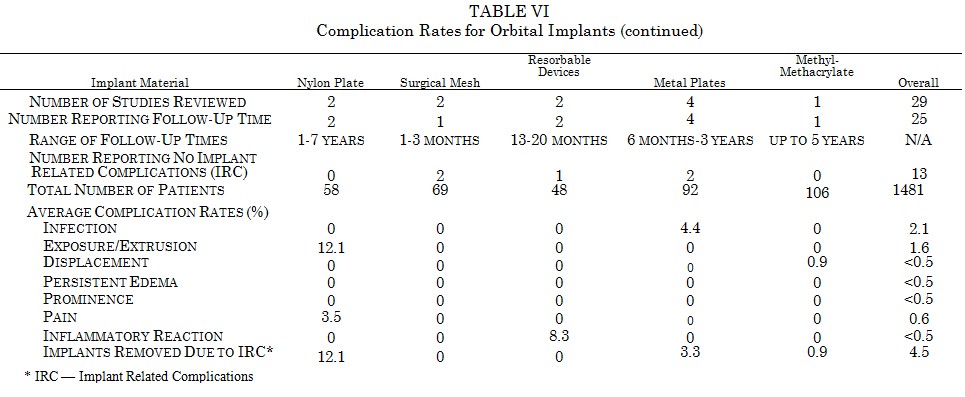
Complication Rates for Orbital Implants
Many materials have been used for implants in the orbital floor. Table V and Table VI show complication rates for polytetrafluoroethylene,164-167 silicone,111,168-173 dense polyethylene,110,174,175 porous polyethylene,143,176 Proplast,123,177 nylon plate,178,179 surgical mesh,180,181 resorbable devices,182,183 metal plates,184-187 and methylmethacrylate.188 High rates of extrusion are noted for the rigid smooth surfaced implants made from silicone (3.1 percent) and nylon (12.1 percent). Metal plates are firmly fixed when used in the orbital floor and tend not to extrude. They do, however, have a 4.4 percent infection rate. Similar to the reports with resorbable plates, biodegradable devices used in the orbital floor manifest an 8.3 percent incidence of sterile inflammatory reaction. Overall, orbital implants have an average infection rate of 2.1 percent and extrusion rate of 1.6 percent.
RTV silicone has been used for volume augmentation in the enophthalmic socket.189-192 The elastomer is mixed with a curing agent, injected into the desired tissue plane, and allowed to harden in situ. Success with this technique has proven to be highly operator dependent, and data from these studies have not been tabulated with the other orbital implant materials. The polymer can shift as it hardens, leading to displacement rates as high as 44 percent.
There are many reports in the literature of late complications with orbital implants, especially with silicone, polytetrafluoroethylene, and nylon plate. These have been noted to occur as late as 21 years after placement, and include infection,193,194 extrusion,195 migration with hematoma formation,196-199 migration with obstruction of the lacrimal duct,200,201 erosion into the maxillary sinus,202-204 and lower eyelid deformity.205,206
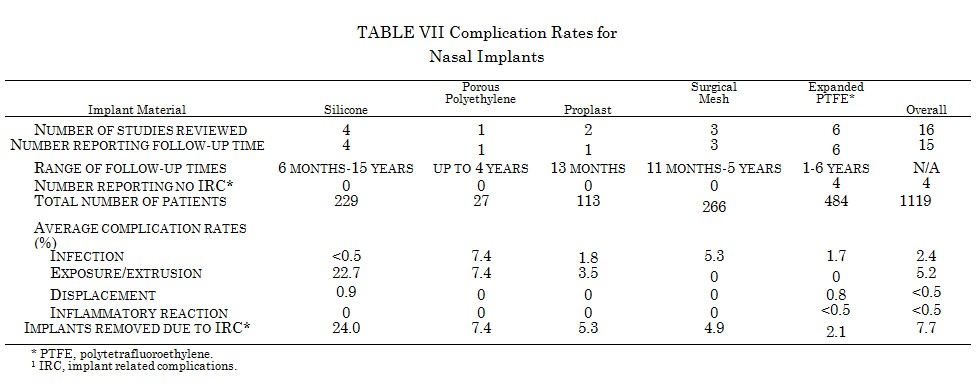
Complication Rates far Nasal Implants
Table VII shows complication rates for silicone,111,207-209 expanded polytetrafluoroethylene,210-215 surgical mesh,216-218 Proplast,123,219 and porous polyethylene.143 The thin soft tissue in this site puts the implant at high risk for extrusion, greatest for silicone devices at 22.7 percent. Although specific numbers are not available, most authors working with silicone implants note that the rates of extrusion are significantly greater with columellar strut implants than dorsal bridge implants. Much lower extrusion rates are noted for more compliant materials such as surgical mesh and expanded polytetrafluoroethylene. Indeed, no extrusions were seen in 266 patients with surgical mesh implants and 484 patients with polytetrafluoro-ethylene implants. The infection rates with these materials were 5.3 and 1.7 percent, respectively. In addition, polytetrafluoroethylene was associated with a small incidence of a transient sterile inflammatory reaction and dis¬placement secondary to implant kinking. The overall infection and extrusion rates for 1119 patients with nasal implants were 2.4 and 5.2 percent, respectively.
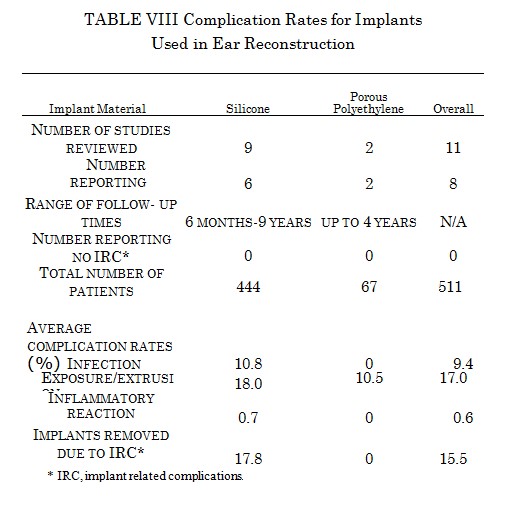
Implants Used in Ear Reconstruction
The largest experience with alloplastic materials in ear reconstruction has been with silicone111,220-227 (Table VIII). As with nasal implants, the thin soft-tissue cover predisposes ear implants to a high rate of extrusion. An extrusion rate of 18.0 percent is seen in 444 patients with these devices. There is a wide variation in the complication rates between different studies. This is in part because clinicians tend to start new series as their tehniques improve. Porous polyethylene has enjoyed greater success than silicone.143,228 An exposure rate of 10.5 percent was seen in 67 patients, and all of these were managed conservatively without removal of the alloplast.
Liquid Silicone Injections
Complication rates for liquid silicone injections in four studies encompassing 747 patients were found to be remarkably low.24,229-231 There was a 1.9 percent incidence of granuloma formation leading to excision in 1.2 percent of patients. Mild inflammatory reactions were seen in 0.1 percent of patients. Clinicians who have used liquid silicone with success argue that complications can be avoided by using the pure form of liquid silicone originally prepared by Dow Corning. Despite these apparent good results, there are reports in the literature of very severe chronic inflammatory reactions after liquid silicone injection.232-235 Many of these patients required extensive surgical debridement. Liquid silicone is not currently approved by the Food and Drug Administration as an injectable implant.
PITFALLS OF ALLOPLASTIC IMPLANTS: THE PROPLAST TEMPOROMANDIBULAR JOINT EXPERIENCE
An important lesson to be learned from clinical experience is that the biocompatibility of a material can vary depending on the under which the implant is placed. Proplast had been used for malar, chin, nasal, and orbital floor implants with acceptable complication rates.
When it was used as an interpositional disc implant in the temporomandibular joint, however, the results were devastating. Although some clinicians reported a low rate of implant removal during long-term follow- up,236,237 others heeded the results of radio- graphic studies showing bony erosion at the temporomandibular joint and removed a high percentage of implants despite the absence of clinical symptoms.238-240 It became apparent that nearly all of the implants fractured over time under the load of the joint. Moreover, the particulate fragments of Proplast would elicit a vigorous foreign body reaction and subsequent erosion of the joint, with occasional perforation into the middle cranial fossa.241-243 Symptoms of pain did not always occur during this process. In 1990, the Food and Drug Administration issued a safety warning urging surgeons to discontinue use of the device.244 Proplast is no longer manufactured in the United States.
DISCUSSION
Implantable biomaterials have a prominent role in plastic surgery of the face and facial skeleton. The broad clinical experience with implants over the past several decades has led to published reports on many thousands of patients, providing a valuable source of information about the safety of these devices and factors that contribute to morbidity.
The incidence of cancer associated with current metal and polymeric implants, especially in craniofacial applications, is extremely low. Only a small number of case reports document cancers in association with implants. The hypothesis that a long latency period is required for tumor development does not appear to be supported by the literature. Indeed, World War I and World War II veterans are not presenting in the 1990s with tumors in association with de facto implants from shrapnel wounds. Hopefully, these low rates of cancer with surgical implants will continue to be supported by objective long-term data as more implants withstand the test of time.
Hypersensitivity reactions are also extremely rare for metal implants and practically unheard of for polymer implants.
Assessing the incidence of systemic disease in association with implant devices, especially au-toimmune disorders, is complicated by the high-profile litigation involving manufacturers of breast implants. Anecdotal evidence provided by patients who allege that their silicone implants caused systemic disease can be quite compelling. To draw a relationship between implants and systemic disease in an objective manner, however, one must first recognize that patients with or without a history of implants may develop an autoimmune disease. Then, one must demonstrate that patients with a history of implants develop autoimmune disease more frequently than those without a history of implants. This bias not been shown to be true;Â objective data reveal that a history of silicone implants does not present an increased risk for developing autoimmune disease.51,52
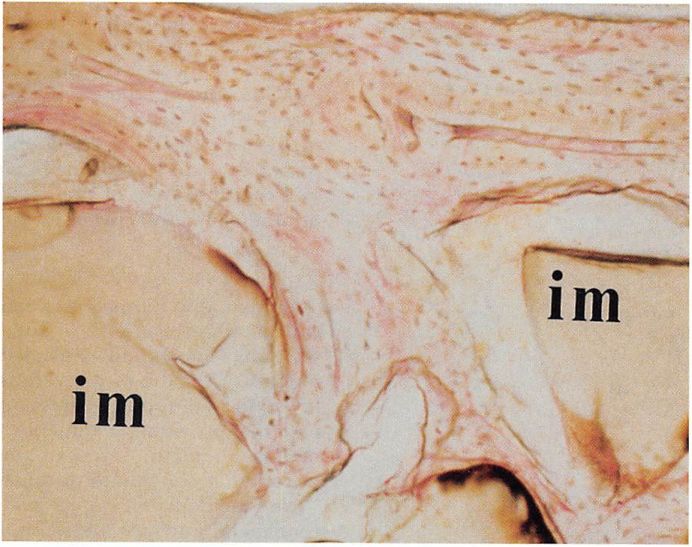
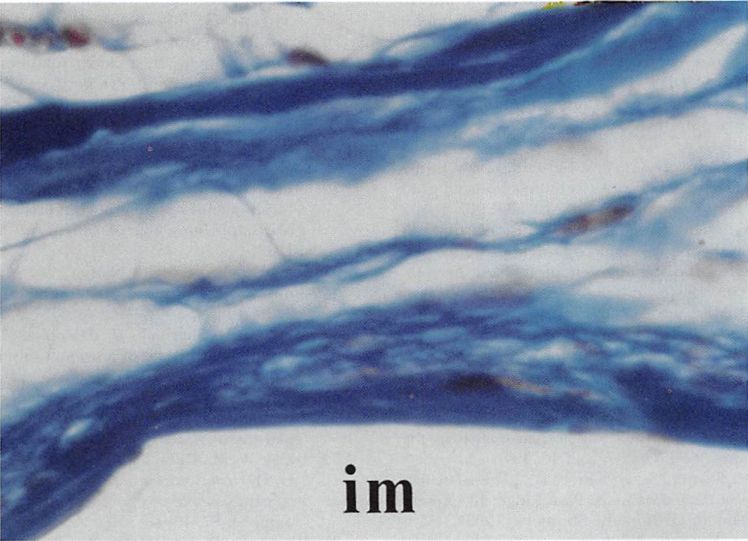
Clinical series and case reports can provide useful information about factors that contribute to morbidity with implants. Implant com¬position is one such factor. Given that several biomaterials are well tolerated in the human body, the actual chemical structure of an implant is important only to the degree that it influences the consistency and surface characteristics of the finished device. For example, porous forms of polyethylene and polytetrafluoroethylene are extruded from the orbital floor much less frequently than smooth surfaced nylon implants. The ingrowth of tissue helps fix the porous implant in place (Figs. 4 and 5). Moreover, experimental data suggest that the presence of host defenses within the implant can decrease the risk of infection.245 Nasal implants provide another example of how implant characteristics affect complication rates. Smooth surfaced rigid silicone implants extrude frequently when used at that site. Expanded polytetrafluoroethylene nasal implants, which are very pliable and allow some tissue ingrowth, are rarely reported to extrude. Few data exist to suggest that the type of metal used in fixation devices influences morbidity, and all metals in current use are highly resistant to corrosion.
Implant site is clearly related to morbidity. Complication rates are lowest in the chin and malar regions, and highest in the nose and ear, where the soft-tissue cover is thin and often under tension from the implanted device. In the ear, the development of flexible implant frameworks has shown promise of lower complication rates than with rigid devices. Similar site-specific morbidity is seen with metal plates. Plates used in the upper facial skeleton, for example, are more likely to become prominent, whereas those used on the mandible are more prone to hardware loosening. Differences in surgical technique also play a role in implant morbidity. Wide variations in complication rates can be seen between authors using the same material in the same site. Implant preparation, perioperative antibiotic regimens, operative approach, and method of implant fixation can all serve as variables affecting implant success.
One striking feature of clinical studies reporting on the use of biomaterials is the variation in follow-up times. Nearly all studies follow patients through the early postoperative period, but early results may mean little for implants placed under load or beneath a thin soft-tissue cover. Using the body of literature to understand implant complications has limitations. First, one must assume that complication rates published in the literature reflect the actual experiences of most surgeons. This may not be true. Some clinicians may be reluctant to publish a series with poor results. In addition, patients with implant failures may be managed by other physicians while being lost to follow-up by the primary surgeon. The importance of careful follow-up and accurate reporting by surgeons cannot be overstressed. Second, one must be cautious when comparing data on implant complications averaged from a number of retrospective studies. This type of analysis suggests trends in complication rates with different materials at different sites, but is not a truly accurate way of comparing materials. Adequate comparison of implant materials would require prospective studies that control for surgical technique, patient selection, and follow-up time. The data derived in this review are most useful when the number of patients in each group is large and when great differences in complication rates are seen between groups. For example, an infection rate of 1.4 percent for all chin implants becomes meaningful when it is based on reports encompassing over 2000 patients.
CONCLUSIONS
Current implant materials have favorable complication rates in most craniofacial applications. No single material, however, is ideal for all applications. We should continue to address the demands of specific implant sites when designing devices for the future.