Tissue Engineering Cartilage with Aged Articular Chondrocytes In Vivo
Background: Tissue engineering has the potential to repair cartilage structures in middle-aged and elderly patients using their own “aged†cartilage tissue as a source of reparative chondrocytes. However, most studies on tissue-engineered cartilage have used chondrocytes from postfetal or very young donors. The authors hypothesized that articular chondrocytes isolated from old animals could produce neocartilage in vivo as well as articular chondrocytes from young donors.
Methods: Articular chondrocytes from 8-year-old sheep (old donors) and 3- to 6-month-old sheep (young donors) were isolated. Cells were mixed in fibrin gel polymer at 40 X 10° cells/ml until polymerization. Cell-polymer constructs were implanted into the subcutaneous tissue of nude mice and harvested at 7 and 12 weeks.
Results:
Samples and native articular cartilage controls were examined histologically and assessed biochemically for total DNA, glycosaminoglycan, and hydroxyproline content. Histological analysis showed that samples made with chondrocytes from old donors accumulated basophilic extracellular matrix and sulfatcd glycosaminoglycans around the cells in a manner similar to that seen in samples made with chondrocytes from young donors at 7 and 12 weeks. Biochemical analysis revealed that DNA, glycosaminoglycan, and hydroxyproline content increased in chondrocytes from old do nors over time in a pattern similar to dial seen with chondrocytes from young donors. Conclusions: This study demonstrates that chondrocytes from old donors can be rejuvenated and can produce neocartilage just as chondrocytes from young donors do when encapsulated in fibrin gel polymer in vivo. This study suggests that middle-aged and elderly patients could benefit from cartilage tissue-engineering repair using their own “aged†articular cartilage as a source of reparative chondrocytes. (Plasl. Reconstr. Sing. 118: 41, 2006.)
Middle-aged and elderly patients frequently require reconstruction and repair of cartilage structures in the face, neck, and joints.1 Although autogenous tissue grafting and insertion of prostheses have been used successfully, these therapies cause morbidity to the patient due to donor-site harvest and/or extrusion of the prosthesis postoperatively.2,3 In addition, the lack of readily available autologous donor tissue (cartilage) limits the spectrum of these therapeutic approaches. The concept of tissue engineering is one of the most promising avenues for creating suitable tissue for repair and regeneration.1’4-5 Using small cartilage biopsy specimens to isolate and expand cells in culture, tissue-engineering techniques could be used to produce large pieces of neocartilage to augment or replace deficient tissue in the recipient, while at the same time significantly reducing donor-site morbidity.
Most published studies on tissue engineering of cartilage in vitro and in vivo have used chondrocytes obtained from fetal or very young donors.’-1′ In vitro studies have shown that chondrocytes isolated from old donors have decreased proliferation, metabolism, and production of cartilage extracellular matrix components (type II collagen, aggrecans, and so on) when compared with chondrocytes from young donors.10-13 In addition, when chondrocytes from old donors have been encapsulated in alginate in vitro, they show a lower production of cartilage extracellular matrix components compared with young cells.” Therefore, articular chondrocytes from old donors could have a diminished capacity to engineer neocartilage in vivo. However, studies demonstrating the in vivo capacity of articular chondrocytes from aged donors to engineer neocartilage are lacking.
Our laboratory and others have demonstrated that chondrocytes from young donors encapsulated in fibrin gel polymer produce extracellular matrix in vivo that resembles native cartilage.1’”17 Furthermore, ii has been shown that neocartilage can be produced in customized shapes, such as the human nose, with fibrin gel polymer in vitro.IS Given its favorable properties and performance in previous studies, fibrin gel polymer can be considered a suitable cell scaffold for tissue-engineering cartilage using chondrocytes from old donors.
The goal of this study was to determine whether articular chondrocytes from old donors encapsulated in fibrin gel polymer can produce neocartilage in vivo comparable to that made with chondrocytes from young donors. This study demonstrates that articular chondrocytes from old donors do indeed form cartilage extracellular matrix similar to that made by chondrocytes from young donors in vivo.
MATERIALS AND METHODS
Cartilage Harvest and Cell Isolation
All procedures involving animals were approved by the Institutional Animal Care and Use Committee of the Massachusetts General Hospital following the National Institutes of Health Guide for the (Care and Use of Laboratory Animals. Articular cartilage was aseptically harvested from the knees, hips, and shoulders of 8-year-old sheep (n = 4) and 3- to 6-month-old sheep (n = 6). Articular cartilage was dissected sharply from the underlying bone. Native articular cartilage samples were collected and set aside as controls. The cartilage chips were washed twice with phosphate-buffered saline (Sigma Corp., St. Louis, Mo.) containing 1% antibiotic antimycodc (Sigma) and minced into 1- to 2-nnn fragments under sterile conditions. Articular cartilage was digested with 0.1 w/v% collagenase type II (Worthington, Freehold, N.J.) for 12 to 18 hours at 37°C. After digestion, chondrocytes were rinsed and washed twice with phosphate-buffered saline. Cell number and viability were determined using the trypan blue exclusion dye test Assembly and Implantation of Chondrocyte/ Fibrin Gel Polymer Constructs
Articular chondrocytes from both old and young donors were suspended in Fibrinogen (Sigma) solution. This solution was mixed with an equal volume of thrombin (0.15 ml each; JPI Jones, Middleton, Wis.) in individual 48-well plate chambers until polymerization to form 0.3-ml volume samples each containing 13.3 X I()() cells. Samples made with chondrocytes from both old and young donors were arranged in three groups: unimplanted (n = 8), implanted 7 weeks (/? = 8), and implanted 12 weeks (n = 8). Unimplanted group samples were stored at —80°G for later analysis for baseline biochemical content. Seven- and 12-week group samples were implanted into the subcutaneous tissue of the backs of nude mice (Massachusetts General Hospital, Boston, Mass.; four samples per animal, eight samples per group). The samples implanted in each mouse were from the same group and donor age. Samples were harvested after 7 and 12 weeks. Both unimplanted and samples implanted for 7 and 12 weeks were randomly selected and processed for either histological examination (n = 2) or biochemical analysis (n = 6). Samples assigned to biochemical analysis were stored at —80°C until needed for processing.
Histological Analysis
After fixation with 10% phosphate-buffered formalin for 24 hours, specimens were embedded in paraffin and sectioned. Serial sections were stained with hematoxylin and eosin and safra- nin-O to evaluate histomorphology and deposition of sulfated glycosaminoglycans, respectively.
Biochemical Analysis
Unimplanted samples and samples harvested after 7 and 12 weeks of in vivo implantation were analyzed biochemically to evaluate the variation in glycosaminoglycan, DNA, and hydroxyproline (collagen) content over time.
Glycosaminoglycan Content
Total glycosaminoglycan content ofcell/polymer constructs was determined using papain digestion and the dimethylmethylene blue dye method.1” Samples were lyophilized for 24 hours and then digested under sterile conditions with papain type III (Sigma) at 125 mg/ml in a buffer of 0.1 M NaH,PO.„ 5 nuM ethylenediaminetetraacetic acid, and 5 mM cysteine hydrochloride at pH 7.0 overnight at 60°G before the dye was added. Dimethylmethylene blue stock solution was made using dimethylmethylene blue, sodium chloride, glycine, sodium azide in IN hydrochloride, and water. A spectrophotometer set at a wavelength of 520 nm was used to measure the optical density of the digested samples. A standard curve was generated using chondroitin 6-sulfate (Sigma). All samples and standards were tested in duplicate. Glycosaminoglycan was measured in micrograms per milliliter and reported as a percentage of wet tissue weight.
DNA Content (Cell Number)
The total DNA content of samples (cell number) was determined using the Hoechst 33258 dye method.20 The dye was used at a concentration of 0.2 mg/ml in 0.01 M Tris, 1 mM EDTA, and 0.1 M sodium chloride. A spectrofluorometer (KO 100 Fluorometer; Hoefer Scientific Instruments, San Francisco, Calif.) was used to estimate the fluorescence of the papain-digested samples, and emission was measured at 400 to 550 nm or an excitation wavelength of 365 nm. Sterile DNA isolated from calf thymus (Sigma) was used to generate standardized curves. DNA content was reported as a percentage of dry tissue weight. The number of chondrocytes per sample can be calculated assuming an average of 7.7 pg of DNA per chondrocyte.20
Hydroxyproline Content (Total Collagen Content)
The hydroxyproline content of the tissue digest was determined as described elsewhere.21 Briefly, the papain digests were hydrolyzed with equal volumes of 6N hydrochloride at 115°C for 16 to 24 hours. Chloramine T hydrate (Sigma) and /;-dimethylaminobenzaldehyde were added to hydrolyzed specimens and absorbancy was detected at 560 nm with a spectrophotometer immediately after the addition of the dye. The hydroxyproline content of the specimens was determined using shark cartilage as the standard (Sigma). All samples and standards were analyzed in duplicate. Hydroxyproline was measured in micrograms per milliliter and reported as a percentage of wet tissue weight.
Statistical Analysis
Differences in mass and biochemical analysis in the tissue-engineered samples made with chondrocytes from both old and young donors at 0 weeks, 7 weeks, and 12 weeks and native cartilage were determined by two-way analysis of variance with Bonferroni’s multiple comparison posttest using the statistical software GraphPacl Prism version 4.00 for Windows (GraphPad Software, San Diego, Calif.). Data are given as mean ± SEM.
RESULTS Gross Examination and Mass
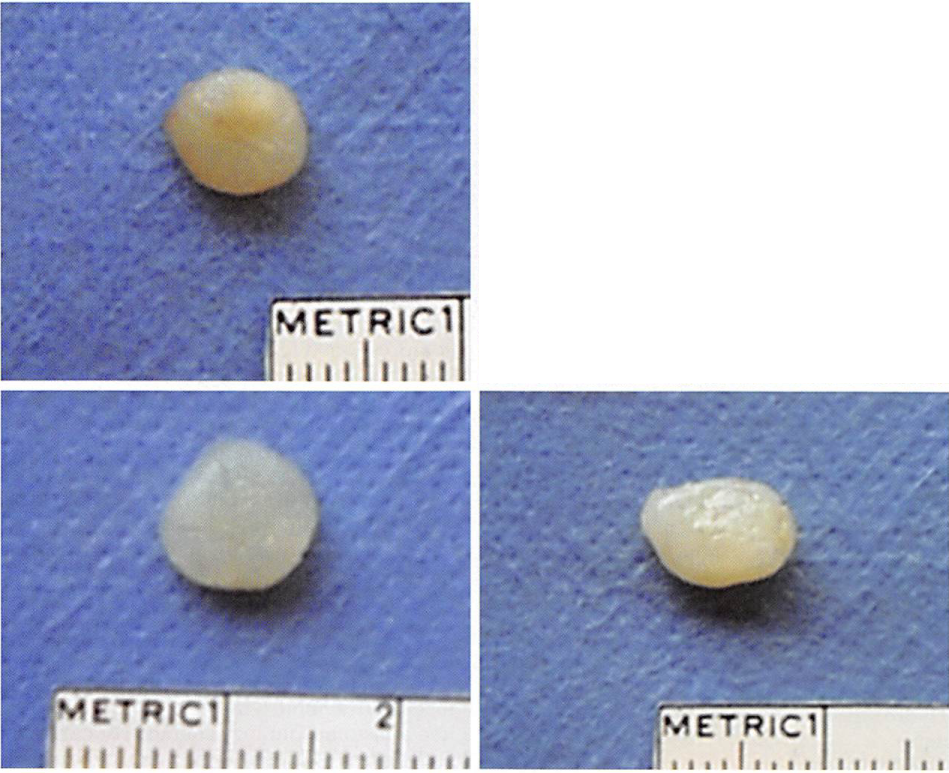
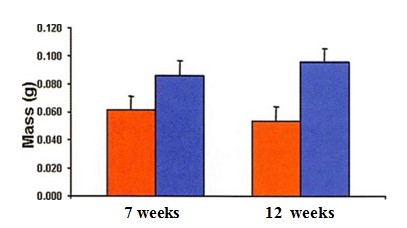
All tissue-engineered samples per group were recovered after in vivo implantation. Harvested samples were surrounded by a thin fibrous capsule that was easily removed. The initial shape was conserved over time in all the experimental constructs. Specimens made with chondrocytes from both old and young donors resembled cartilage in color and texture and were resistant to external compression (Fig. 1). The mean mass of the engineered samples made with chondrocytes from old donors decreased slightly from 7 weeks to 12 weeks, while the mass of samples made with chondrocytes from young donors increased slightly (Fig. 2). However, these changes were not statistically significant (/;> 0.05). At 12 weeks, the mass of samples made with chondrocytes from young donors was significantly higher than the mass of samples made with chondrocytes from old donors (p < 0.05).
Histological Examination
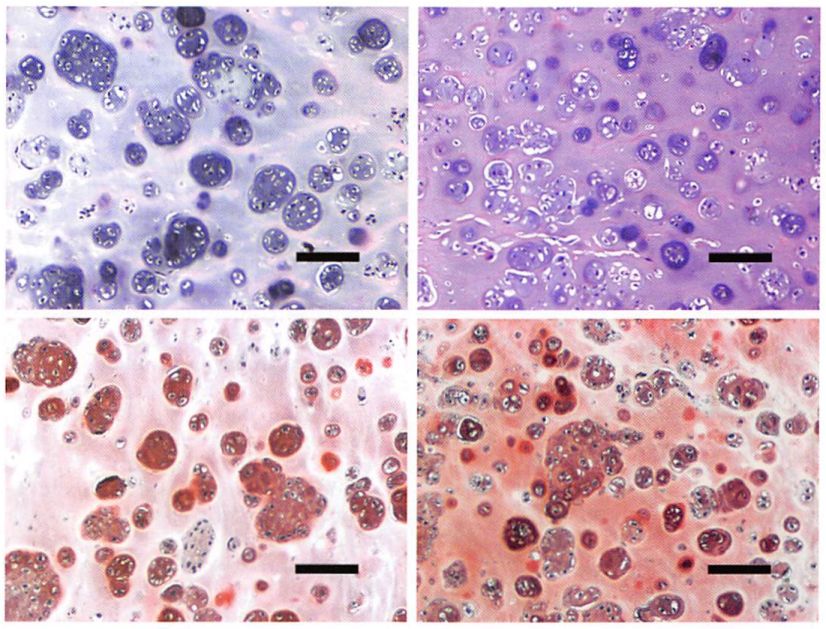
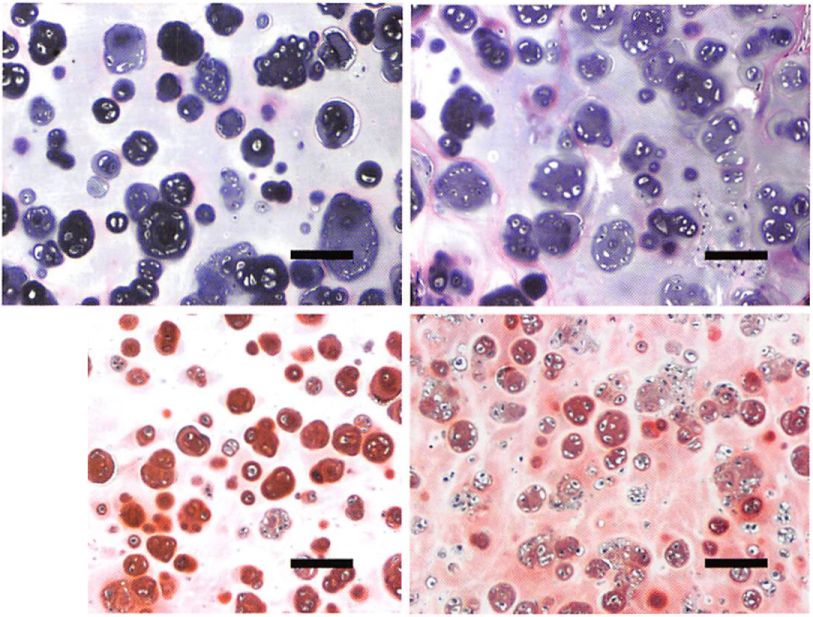
Hematoxylin and eosin staining of samples made with chondrocytes from old donors exhibited a histomorphology characteristic of cartilage (cells within basophilic ground substance) at 7 and 12 weeks after implantation (Fig. 3). Deposition of glycosaminoglycans was also evident with safraninO staining at both time points (Fig. 3). The extracellular matrix produced by chondrocytes from old donors was similar to that made by chondrocytes from young donors; however, no sample produced contiguous neocartilage across the entire specimen at the experimental time points studied.
Biochemical Analysis
Glycosaminoglycan Content
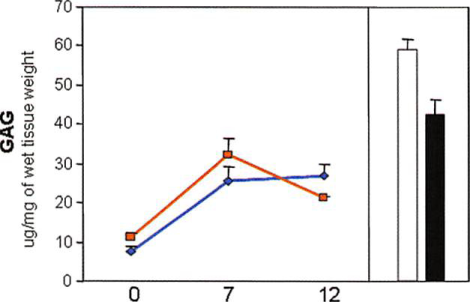
The glycosaminoglycan content in native articular cartilage as a function of donor age was significantly different. Native articular cartilage from old donors contained 28 percent less glycosaminoglycan than native cartilage from young donors did (p < 0.05) (Fig. 4, above). Analysis of glycosaminoglycan content in samples engineered with chondrocytes from old donors showed an increased production from before implantation (time 0) to 7 weeks (p < 0.05) and a slight decrease by 12 weeks that was not statistically significant over time (p > 0.05). At 12 weeks, the glycosaminoglycan content of samples reached 48 percent of the native cartilage value (old donors). No significant differences were observed in glycosaminoglycan content in samples made with chondrocytes from both old and young sources at the experimental time points (p > 0.05).
Fig. 1. Gross appearance. (Above, left and right) Neocartilage made with chondrocytes from old donors after 7 and 12 weeks of implantation, respectively. (Below, left and right) Neocartilage made with chondrocytes from young donors after 7 and 12 weeks of implantation, respectively.
DNA Content
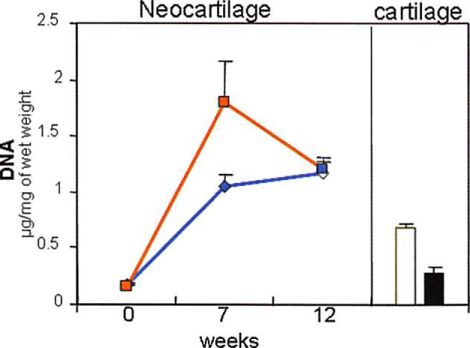
The DNA content (cell number) in native cartilage also showed significant differences as a function of donor age. Native articular cartilage from old donors contained 54 percent less total DNA content than native cartilage from young donors (/; < 0.05) (Fig. 4, center). The average cell yield was 9.6 X 10s ± 1.4 X 10:$ cells/mg for native cartilage from old donors and 25 X 10s ± 3.9 X 101 cells/mg for native cartilage from young donors. The DNA content of samples made with chondrocytes from both old and young donors increased significantly from before implantation to 7 weeks. At 7 weeks, the DNA content of engineered samples made with chondrocytes from old donors was significantly higher than the content of samples made with chondrocytes from young donors (/; < 0.05). By 12 weeks, the DNA content of samples made with chondrocytes from old donors decreased significantly, while the DNA content of samples made with chondrocytes from young donors increased slightly.
Fig. 2. Mean mass of neocartilage produced with chondrocytes from old (redbars) and young (blue bars) donors after 7 and 12 weeks of in vivo implantation. Data are presented as mean ± SEM.
However, at this time point (12 weeks), there was no significant difference in the DNA content of samples made with chondrocytes from both old and young donors (/; > 0.05). The DNA content of engineered samples at 12 weeks was 4.1 times the DNA content of native young cartilage for samples made with chondrocytes from young donors and 1.7 times the DNA content of native old cartilage for samples made with chondrocytes from young donors.
OLD CHONDROCYTES
YOUNG CHONDROCYTES
Fig. 3. Histological evaluation of tissue-engineered samples. Samples made with chondrocytes from old donors [above) and young donors (below) after 7 weeks (left column) and 12 weeks (right column) of in vivo implantation were stained with hematoxylin and eosin for evaluation of morphology and with safranin0 for evaluation of glycosaminoglycan deposition (bar = 100 /xm).
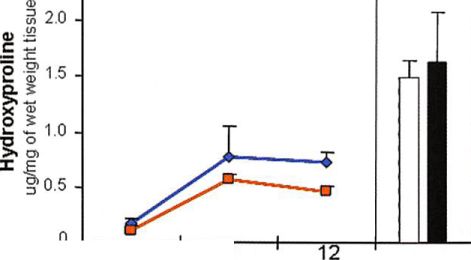
Fig. 4. Biochemical analysis: (above) glycosaminoglycan content (GAG), (center) DNA content, and (below) hydroxyproline content of chondrocytes from old and young donors encapsulated in fibrin gel before implantation (0 weeks to unimplanted) and after in vivo implantation (7 and 12 weeks) (line graphs: tissue-engineered young, blue lines, tissue-engineered old, red lines), and native articular cartilage from old and young donors (bars: native young,  native old, ). Data are presented as mean ± SEM.
Total Collagen Content
The hydroxyproline content (total collagen content) in native articular cartilage did not show a significant difference with donor age (/; > 0.05) (Fig. 4, below). The tissue-engineered samples made with chondrocytes from old donors showed a production of collagen by 7 weeks that was unchanged at 12 weeks. At 12 weeks, the total collagen content reached 32 percent of the amount found in native cartilage from old donors (/; < 0.05). Samples made with chondrocytes from young donors showed similar total collagen production at the specified time points (/; > 0.05).
DISCUSSION
The results from this study indicate that articular chondrocytes from old donors do in fact have the capacity to be rejuvenated when removed from their native matrix and to generate tissue-engineered cartilage when encapsulated in fibrin gel polymer in vivo. In general, as demonstrated by histological and biochemical analyses, engineered neocartilage produced with articular chondrocytes from old sources has characteristics similar to those of neocartilage made with articular chondrocytes from young donors. Thus, the data generated from this study suggest that it would be possible to use autologous articular chondrocytes to engineer neocartilage for reconstruction and repair in the elderly population.
Previously published studies suggested that articular chondrocytes isolated from “aged†human and animal cartilage sources could have an impaired capacity to produce cartilage matrix in vivo.22″-’’ Kamada et al. showed that when bovine articular chondrocytes isolated from fetal, young, and old animals were cultured in alginate beads, proteoglycan production was inversely proportional to age.” Our results using fibrin gel scaffold in vivo contradict their in vitro findings. We found that the production of glycosaminoglycans in the in vivo tissue-engineered samples increased over time and did not show dependence based on donor age. In addition, Rotter et al. also found that human nasal chondrocytes from old donors seeded onto polylactic acid/polyglycolic acid scaffolds produced neocartilage in vivo similar to that generated by chondrocytes from young donors.21’ These results suggest that the type of cell scaffold could have a strong influence on the behavior of the chondrocytes, since chondrocytes from old and young donors produced similar quantities of glycosaminoglycan when encapsulated in either fibrin gel or PGA/PLA scaffolds in vivo. However, the intrinsic differences between the in vitro conditions used by Kamada et al. and others and the in vivo conditions used in our experiments also could have a strong influence on glycosaminogly can production, independent of the type of scaffold used. In addition, these data suggest that the “juvenile†regenerative capacity of chondrocytes from old donors is not limited to a single anatomical cell source.
Studies by Martin et al. and others have shown that chondrocytes from aged articular cartilage have a decreased mitotic index (cell multiplication) and increased expression of senescence-associated markers with age.ll,22,2:5-2′ Our study demonstrated that chondrocytes from old donors increased in cell number over time, as evidenced by the increase in DNA content of samples from preimplantation values to 12-week values. Interestingly, by 12 weeks, the DNA content of these samples was several times higher than the DNA content found in unimplanted samples (two times) and native cartilage from old donors (4.1 times). These results suggest that the release of chondrocytes from their aged native environment (matrix) and incorporation in fibrin gel scaffold may play an important role in restoring the replicative capacity of “senescent†chondrocytes from old donor sources. However, the significant decline in cell number in samples made with chondrocytes from old donors from 7 to 12 weeks, associated with a slight decline in mass, suggests that some cells could have undergone apoptosis, since histological analysis did not show evidence of inflammation or necrosis. Therefore, further studies with a longer time frame should be performed to evaluate this phenomenon and possible implications in the maintenance of neocartilage mass and volume over time. Collagen, especially type II collagen, is one component of the cartilage extracellular matrix that defines the type of cartilage (hyaline versus fibrocartilage) and is an important constituent contributing to the biomechanical properties of native and engineered cartilage. Our study shows that although the total collagen content (hydroxyproline content) found in samples made with chondrocytes from old donors was similar to that found in samples made from young cells, it was very low compared with the values found in native cartilage (<50 percent). These results were in accordance with most of the published data obtained regarding tissue engineering cartilage with chondrocytes from young donors and different anatomical sources.28’2’1 Therefore, it seems that the low production of collagen by chondrocytes is a fundamental problem of tissue engineering cartilage and not specifically dependent on donor age.
The current study showed that only tissue-engineered samples made with chondrocytes from old donors slightly decreased in mass from 7 to 12 weeks. This finding was not associated with a decrease in glycosaminoglycan or total collagen content (hydroxyproline), both of which increased over time (Fig. 4, above and below). This change in mass could have been associated with a change in the structure of the glycosaminoglycan produced. Several published studies have shown that the length and number of chondroitin sulfate and keratan sulfate chains attached to hyaluronic acid (structures that retain water in the glycosaminoglycan) decrease with increasing age.1014 Therefore, the type and characteristics of glycosaminoglycan produced by chondrocytes from old donors when tissue engineering cartilage in vivo should be studied to determine their implications in the retention of mass over time and the biomechanical properties of the neocartilage produced with these cells.
Our laboratory has previously shown that articular chondrocytes from young swine donors produce homogeneous extracellular matrix at 12 weeks, using similar experimental conditions.’b,3H In this experiment, the extracellular matrix of the tissue-engineered samples made with chondrocytes from old sheep donors was similar to that of samples made with chondrocytes from young donors. However, the distribution of new matrix was not contiguous throughout the samples at 12 weeks. Panossian et al. demonstrated that chondrocyte concentration influences the rate of extracellular matrix production over time.”’ Since there is a difference in the cartilage extracellular matrix production by chondrocytes from different animal models using equivalent cell/polymer scaffold concentrations, the effects of different variables, such as cell concentration, type of cell scaffold, time of in vivo implantation, and so on, should be addressed to fully understand the engineering of neocartilage with sheep chondrocytes. Similarly, the optimal matrix-forming parameters of cells from old human donors will need to be worked out as well.
Our study shows that there is a significant difference in the DNA content of native articular cartilage based on donor age. Native articular cartilage from old donors contains 54 percent fewer cells per gram of tissue than native articular cartilage from young donors. This difference suggests that to obtain a certain number of chondrocytes from a native articular cartilage biopsy sample from old patients, the size should be twice that of the biopsy sample obtained from young patients. Clinically, the necessity of harvesting bigger samples of cartilage in the elderly population for tissue-engineering purposes may increase the donor site morbidity associated with the articular cartilage harvest.31 However, further studies evaluating the capacity of culture-expanded articular chondrocytes from old donors should be performed to provide a more accurate estimate of the cartilage biopsy size (cell number) required to be harvested from old patients for tissue-engineering purposes.
CONCLUSIONS
Articular chondrocytes from old sheep donors produce tissue-engineered cartilage in vivo. Chondrocytes from old donors encapsulated in fibrin gel polymer can be rejuvenated and produce neocartilage in vivo with characteristics similar to those of the neocartilage made with young donors, as evidenced by gross appearance and histological and biochemical analysis. This study suggests that middle-aged and elderly patients could benefit from cartilage tissue-engineering approaches using their own “aged†autologous articular cartilage as a source of reparative chondrocytes.
ACKNOWLEDGMENTS This study was supported by a grant from the Plastic Surgeiy Educational and Research Foundation and the 2003 Lyndon Peer Fellowship Award sponsored by the Plastic Surgeiy Educational Foundation and the American Society of Plastic Surgeons. The authors thank Arthur Foubert for kindly providing the young sheep.
REFERENCES
1. Danger, R., and Vacanti,). P. Tissue engineering. Science260: 920, 1993.
2. Ohara, K., Nakamura, K., and Olita, E. Chest wall deformities and thoracic scoliosis after costal cartilage graft harvesting. Plfisl. Recanstr. Surg. 99: 1030, 1997.
3. Rubin, J. P., and Yaremcluik, M. J. Complications and toxicides of implantable biomaterials used in facial reconstructive and aesthetic surgeiy: A comprehensive review of the literature. Rlast. Rrconstr. Surg. 100: 1336, 1997.
4. Lalan, S., Pomerantscva, I., and Vacanti, J. P. Tissue engineering and its potential impact on surgery. World J. Surg. 25: 1458,2001.
5. Vacanti, G. A., and Vacanti. J. P. The science of tissue engineering. Ortho/). Clin. North Aw. 31: 351, 2000.
6. Passaretli, I)., Silverman, R. P., Huang, W., et al. Cultured chondrocytes produce injectable tissue-engineered cartilage in hydrogel polymer. Tissue Eng. 7: 805, 2001.
7. Perka, C., Scluiltz, O., Lindenhayn, K.. et al. Joint cartilage repair with transplantation of embryonic chondrocytes embedded in collagen fibrin matrices. Clin. Exp. Rheumatol. 18: 13, 2000.
8. Fuchs, J. R., Terada. S.. Hannouchc, D., et al. Engineered fetal cartilage: Structural and functional analysis in vitro. J. Bediatr. Surg. 37: 1720, 2002.
9. Rodriguez, A., Cao, V. 1… Ibarra, (‘… et al. Characteristics of cartilage engineered from human pediatric auricular cartilage. Hast. Reconstr. Surg. 103: 1111, 1999.
10. Verbruggen, G., Cornelissen, M., Almqvist, K. F.. et al. Influence of aging on the synthesis and morphology of the aggrecans synthesized by differentiated human articular chondrocytes. Osteoarthritis Cartilage 8: 170, 2000.
11. Martin, J. A., and Buckwalter, J. A. The role of chondrocyte senescence in the pathogenesis of osteoarthritis and in limiting cartilage repair./. Bone joint Surg. (Am.) 85 (Suppl. 2): 106, 2003.
12. Parsch, D., Brummendorf, T. H., Richter, W., and Fcllenberg, J. Replicative aging of human articular chondrocytes during ex vivo expansion. Arthritis Rheum. 46: 2911. 2002.
13. Dozin, B., Malpeli, M., Camardella, L., Cancedda, R., and Pielrangelo, A. Response of young, aged and osieoartlu itic human articular chondrocytes to inflammatory cytokines: Molecular and cellular aspects. Matrix Biol. 21: 449, 2002.
14. Kamada, I I., Masuda, K., D’Souza, A. L., et al. Age-related differences in the accumulation and size of hyaluronan in alginate culture. Arch. Biorliem. Biophys. 408: 192, 2002.
15. Silverman, R. P.. Passaretti, D., I luang, W., Randolph, M. A., and Yaremcluik, M. J. Injectable tissue-engineered cartilage using a fibrin glue polymer. Blast. Reconstr. Surg. 103: 1809, 1999.
16. Panossian, A.. Ashiku. S., Ivirchhoff, C. II., Randolph, M. A., and Yaremcluik, M. J. Effects of cell concentration and growth period on articular and ear chondrocyte transplants for tissue engineering. Blast. Reconstr. Surg. 108: 392, 2001.
17. van Susante, J. I.., Buma, P., Sclmman, I.., et al. Resurfacing potential of heterologous chondrocytes suspended in fibrin glue in large fullthickness defects of femoral articular cartilage: An experimental study in the goal. Biomaterials 20:
1 167, 1999.