Principles of Scalp Surgery and Surgical Management of Major Defects of Scalp
Major defects of the scalp are most often the result of trauma, radiation necrosis, or extirpation for tumor. This chapter presents plastic surgical concepts and techniques used to reconstruct major defects of the scalp.
Throughout history, advances in plastic surgery have been reflected in the management of scalp wounds. Hippocrates, Gallen, and Celsus described the treatment of a denuded skull by perforating the dry black sequestrum formed above bare cranium with an awl. 1 In the 17th century, Augustin Belloste advocated perforating the outer table of the skull to permit granulation tissue formation and subsequent epithelialization. 2 In 1871, Netolitzky described the technique of skin grafting granulating scalp wounds, 3 and in 1908, Robinson described the use of skin graft on intact periosteum. 4 In 1953, Kazanjian ï¬rst described the use of superï¬cial incisions through the galea to increase tissue availability. 5 In 1967 and 1971, Orticochea published his four- and three-fl aps technique, respectively, for coverage of large scalp defects.6,7 Miller, in 1976, replanted microsurgically for the ï¬rst time an avulsed scalp. 8 In 1978, Radovan was the ï¬rst to report successful clinical applications of tissue expansion and to demonstrate prototypes of the expanders in clinical use today. 9-11
Anatomy of the Scalp
The scalp consists of ï¬ve distinct anatomic layers. Listed from the most superï¬cial to the deepest, these layers include: (1) the skin with its characteristic thick dermis; (2) the subcutaneous tissue; (3) the relatively rigid galea aponeurotica, which is continuous with the superï¬cial musculoaponeurotic system, frontalis, occipitalis, and superï¬cial temporal fascia; (4) underlying areolar tissue; and (5)skull periosteum.
The relatively poor ï¬xation of the galea to the underlying periosteum of the skull provides little resistance to shear injuries, resulting in large flaps or “scalping†injuries. This layer’s resultant potential space also provides little resistance to hematoma or abscess formation. As a result, extensive fl uid collections related to scalp injury tend to accumulate in the subgaleal plane.
The rich vascular supply of the scalp receives vascular contribution from the internal and external carotid arteries. The forehead and anterior scalp are mainly supplied by terminal branches of the ophthalmic artery, which is a branch of the internal carotid artery: the supratrochlear and supraorbital arteries. The supratrochlear vessels arise 1.6 to 2.3 cm from the midline, which is usually located at the medial border of the eyebrow. 12 The supraorbital vessels arise through the supraorbital notch which is located 2.4 to 2.9 cm from the midline. 13 The external carotid artery provides three branches to the scalp. The terminal branch of the external carotid artery, the superï¬cial temporal artery, ascends anterior to the helix within the superï¬cial temporal fascia and it supplies the temporal and parietal region. The posterior auricular artery ascends between the auricle and mastoid process and supplies blood to the scalp posterior to the auricle. The occipital artery, which supplies blood to the back of the scalp, pierces the fascia connecting the cranial attachment of the sternocleidomastoid and trapezius muscle, and ascends at a mean distance of 4.2 cm from the midline of the external occipital protuberance. 14
In the subcutaneous layer, there is an abundant communication of vessels, which can result in signiï¬ cant blood loss when the scalp is lacerated.
Planning Scalp IncisionsÂ
The single most important means of preventing or minimizing wound healing complications of the scalp is the thoughtful planning of the initial scalp incision. Because the current treatment of malignant brain tumors commonly requires repeated craniotomies, scarring and a loss of tissue elasticity can be predictably anticipated. Soft tissue is frequently further compromised by external beam radiation, resulting in scalp wound breakdown, failure to heal, or skin necrosis. Therefore, in anticipation of such compromised wound healing, the initial operative incision should be placed so as to maximize blood fl ow to the healing incision, and to allow for alternate salvage maneuvers in the event of wound breakdown. Generally, linear scalp incisions that run parallel to the major arteries of the scalp are preferred over traditional U-shaped craniotomy flaps. Such linear incisions are well perfused, heal favorably, and offer broader surgical options in the event of wound breakdown. 15 Ideally, the scalp incision lies adjacent to, rather than directly over, underlying anticipated cranial osteotomies, microplates, and screws, to avoid hardware exposure and infection of the craniotomy bone segments in the event of minor incisional dehiscence.
Lacerations of the ScalpÂ
Scalp wounds should always be inspected for underlying fractures. Because the scalp has a rich vascular supply, lacerations may result in signiï¬cant blood loss, particularly in children. The large diameter of the scalp vessels often requires that they be individually clamped and ligated for control. Bleeding from a scalp wound can be controlled emergently until deï¬nitive care is provided with temporary pressure dressings, Raney clip placement of the wound edges, or an over-and-over continuous stitch with large sutures.
The rich intercommunicating blood supply of the scalp is such that one set of superï¬cial temporal vessels alone may nourish the entire scalp. This rich vascularity allows the survival of large, narrow-pedicled avulsion flaps that would never survive anywhere else on the body. For that reason, almost all traumatic scalp flaps should be appropriately cleansed, minimally debrided, and anatomically replaced. Only the hair adjacent to the lacerated edges needs to be shaved to allow easier suture placement and removal. The subgaleal space is drained with a closed suction drainage system before the scalp is closed in layers. In general, the galea and the dermis are closed with 2-0 or 3-0 absorbable sutures before the skin is closed with running or interrupted 3-0 or 4-0 nylon sutures.
Wounds with Tissue LossÂ
The management of scalp wounds with soft-tissue loss is determined by the amount of soft tissue lost and the type of tissue exposed. Even relatively small scalp defects can present a reconstructive challenge. The inherent inelasticity of the galea aponeurotica contributes to a property known as “stretch-back,†the tendency for the scalp to contract back toward its original state. 16 Stretch-back leads to increased tension and ischemia across the healing incision, with sequelae ranging from alopecia and widened scars, to nonhealing wounds and tissue necrosis. The convex curvature of the cranium also complicates scalp closures, requiring additional flap length to achieve the desired tissue advancement. Finally, although local scalp flaps provide the best cosmetic outcome, hair-bearing scalp is a limited resource, and one that can be further depleted by pre-existing scars across the axial blood supply.
Local Advancement (Galeal Scoring)
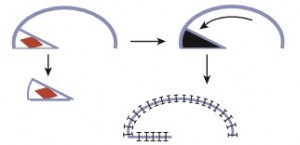
The surface area of the scalp adjacent to the defect can be enlarged considerably by galeal scoring to allow closure by advancement of the wound edge. Each cut, which is made at 1-cm intervals in a parallel or crosshatch fashion, allows the scalp to be stretched approximately 4 to 6 mm. Galeotomies should be oriented perpendicular to the desired direction of advancement, and must be done carefully so as to avoid compromising the vascular supply to the scalp with incisions that are unnecessarily deep. This scoring maneuver requires a complete division of the substantial galeal layer ( Fig. 139-1Â Â Â Â ). Tissue loss of more than 1 to 2 cm may require extension of the laceration to allow greater undermining and scoring. Defects that are 3 cm in diameter can be routinely closed with this technique.
Skin Grafts
Wounds in which soft-tissue loss is so extensive that the skin edges cannot be approximated are closed with either skin grafts or flaps. Full-thickness skin grafts contain the epidermis and the complete thickness of dermis from the recipient area. Split-thickness grafts contain the epidermis and a variable thickness of the dermis. Grafts with a greater thickness of dermis contract less on the wound bed and provide more durable coverage. Thin grafts have the advantage of more rapid revascularization, so they are more likely to be successful. Thin grafts, however, tend to provide less durable coverage.
A skin graft may cover any scalp wound that has capillary circulation, which will ultimately provide a source of vascular ingrowth for that graft. For that reason, skull periosteum or any more superï¬cial scalp layer will support a skin graft. Most scalp defects are closed with thin (0.010- to 0.014-inch thickness), “meshed†skin grafts.
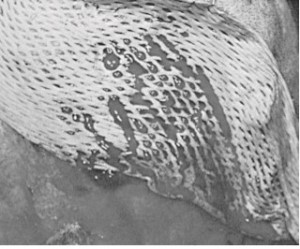
Meshed grafts are those that are mechanically perforated in a grid pattern, which allows them to be expanded and to conform to irregular surfaces. The perforations also provide egress for wound drainage. The resultant improved graft bed contact optimizes conditions for graft take ( Fig. 139-2Â Â Â Â ). For scalp defects, meshed grafts should not be perforated and expanded more than 1.5 times their normal size unless donor skin is in short supply. A widely expanded graft is less desirable because larger open areas take longer to epithelialize and provide poorer protective coverage.
Skin grafts are most easily harvested with an electric dermatome. The upper lateral thigh has skin of sufï¬cient thickness to allow uncomplicated healing of the donor site. Donor site scarring in this area is usually covered with clothing.
To ensure optimal graft-bed interface for graft take, the graft should be immobilized to the scalp recipient site. A tieover stent dressing or a quilt dressing in which the overlying dressing is sutured to the intact wound edges is most often used to achieve this immobilization.
Several flap designs allow closure of scalp wounds with signiï¬cant soft-tissue loss. However, the wound should not be enlarged considerably to close a large traumatic wound emergently; rather, if the periosteum remains, a split-thickness skin graft should be applied. When scalp with periosteum is lost, a closed wound is obtained most expeditiously if, at the time of presentation, the outer table is drilled down to ï¬ nd bleeding points that can provide a bed for a split-thickness skin graft take. A meshed, nonexpanded, split-thickness skin graft is placed immediately at the time of the drilling. An older, well-proven technique involves removing the outer table of the skull to expose the diploë and treating the wound with wet dressings for 5 to 7 days, at which time luxuriant granulation tissue usually forms. This granulation tissue readily accepts a skin graft.
Another method involves making small drill holes 1 cm apart through the outer table down into the diploë space. Usually, granulation tissue arises from these holes and grows over the exposed calvarium to coalesce and form a suitable bed for skin grafting.
 FIGURE 139-1
Scalp flaps can be enlarged considerably by galeal scoring. The galea is completely divided at 1-cm intervals. A, Defect before debridement. B, Defect after debridement. C, Appearance of galea before scoring. D, Appearance of galea after scoring. Note the increase in surface area. E, Resultant closure.  Â
  Recently, multiple studies showed that vacuum-assisted closure devices hasten soft-tissue contraction as well as granulation tissue formation in various trunk and extremity wounds. 17 In the scalp, two small studies demonstrated a possible role for the vacuum-assisted closure device. Molnar et al. treated four patients with exposed skull by removing the outer table, immediate application of a split thickness skin graft to the diploe and treatment of the wound with a vacuum-assisted closure device for 3 to 4 days. The author reported a 100% graft take without complications. 18 In a another study, Umesh et al. reported a successful case report where the author closed a 10 × 12 – cm wound with exposed dura by using the vacuum-assisted closure device over the dura for a period of 3 weeks and then grafting the granulated wound bed with a split thickness skin graft. 19
FIGURE 139-2
A meshed skin graft placed minimally expanded over a scalp wound. Note how perforations in the graft allow egress for drainage from the wound bed.
  Skin grafting directly onto bone is susceptible to breakdown after minimal trauma and leaves an area with alopecia and signiï¬cant contour deformity. This problem can be corrected as a delayed reconstruction with advancement or rotational flaps after galeal scoring or with tissue expanders.   Flaps Wounds with exposed vital structures or wounds that do not have exposed capillary circulation require flap coverage. Whereas skin grafts provide only thin coverage and depend on the wound bed for their revascularization and survival, flaps carry their own blood supply and provide soft-tissue bulk to the wound.
The two basic principles of flap coverage follow: 1 move available tissue with its intact circulation from an area of relative excess to the area of deficiency, and 2 optimize vascularity of the flap. In the scalp, the lateral and posterior aspects are usually used as donor sites to avoid distortion of the forehead or frontal hairline. The flaps are designed to include axial vessels in their bases. Usually, the superficial temporal artery or the occipital artery provides the basic blood supply to these flaps. The flaps should be designed with respect to previous incisions, which may block vascular inflow to the flap. Many types of flaps are possible in the scalp. The most commonly used flaps include rotation flaps and bipedicle advancement flap. 20 Orticochea flap and double-scalping flap are occasionally used for large defects. The flap’s design requires considerable expertise and extensive mobilization of the scalp tissues. Usually most of the scalp must be degloved in the subgaleal space, and the galea should be scored to allow primary closure of the secondary defect.
Rotation flaps use adjacent tissue rotated in an arc to close the defect. An isoceles triangle is designed around the defect, making the shortest side the base of the triangle. Next, a semicircular arc is outlined by creating a half circle that incorporates the triangular base at one end. The length of the flap’s arc are four to six times the size of the base, i.e., the diameter of the defect ( Fig. 139-3).
FIGURE 139-3
Rotation flap. Defect is made in triangular shape, and base of the triangle is incorporated into the rotation arc. Length of flap arc should be at least four times the size of triangle base.  Â
In the scalp, rotation flap are generally used to transfer an anterior hairline defect posterior. In general, a skin graft is needed to close the posterior donor site ( Fig. 139-4 ).
Advancement flaps slide adjacent tissue to close the defects. In the scalp, bipedicle flaps are the most commonly used advancement flaps. They are generally used to close large parietal defects. These flaps are designed to allow advancement into the adjacent defect in a vector that is perpendicular to the flap axis. They are created by making a parallel incision to the longitudinal axis of the wound. The flap length-to-width ratio of 4:1 may be achieved. The occipital and supraorbital arteries are generally incorporated in the design of the flap for parietal wounds. The flap, between the wound edge and the skin incision, is undermined in a subgaleal plane. The fl ap is then advanced to close the defect. At most, the bipedicle advancement flap could be advanced a distance equivalent to the width of the flap. In most cases, back-grafting of the donor site is required ( Figs. 139-5 and 139-6 ; see case report 1).
Orticochea initially described his flap in 1967 and then modiï¬ed in 1971. The Orticochea flap is valuable for large anterior or occipital defect, i.e., greater than 50 cm 2 . In this technique, the remaining scalp is divided into three large flaps. Two flaps are based on the superï¬cial temporal vessels and one flap is based on the occipitals vessel. The Orticochea technique relies heavily on scoring the galea of the three flaps to expand the size of the flap and help close the defect. 21
Recently, Papadopoulos et al. described the double scalping flap for coverage of large full-thickness defect of the vertex. 22 Two large posterolateral flaps are created based on the superï¬cial temporal vessels. The flaps are then rotated anteriorly to cover the defect. A spilt-thickness skin graft is used to cover the donor site ( Fig. 139-7 ).
In brief, good design of the above flaps entails closure of the defect without tension, incorporation of major vascular pedicles and preservation of the native hairline. Subsequent tissue expansion can be used to remove the skin grafted donor site. Wounds too large to allow local flap coverage usually require coverage with a free tissue transfer (free flap).
FIGURE 139-4
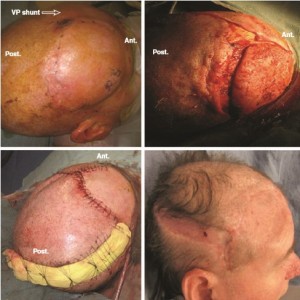
A 38-year-old man with a history of tuberous sclerosis was referred for treatment of exposed methylmetacrylate and titanium mesh in the right parietal and central frontal area. The patient underwent tumor excision, reconstruction with titanium mesh and methylmetacrylate and radiation therapy 20 years ago. He also has presently a ventriculo-peritoneal (VP) shunt in the left parietal area ( upper left ). The methylmetacrylate and titanium mesh were removed using previous scalp incisions. The size of the wound expanded due to the extremely atrophic skin in the area of his previous surgery and radiation (upper right). A large rotation fl ap was done to close the defect. The resultant secondary defect located in the right parietal/vertex area was skin grafted on the skull periosteum with a meshed, split-thickness skin graft that was harvested from the left lateral thigh. This graft was meshed 1 to 1.5 times and placed nonexpanded on the secondary skull defect (lower right). Two months postoperatively ( lower right ). Ant., anterior; Post., posterior. Â
 FIGURE 139-5
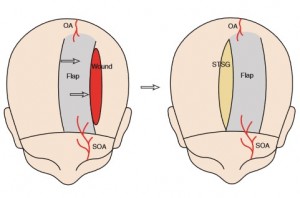
The design of left bipedicled fronto-occipital scalp flap to close a left parietal area wound. The flap should incorporate the left supraorbital (SOA) and occipital arteries (OA). The secondary defect is closed with a split thickness skin graft (STSG).Â
FIGURE 139-6
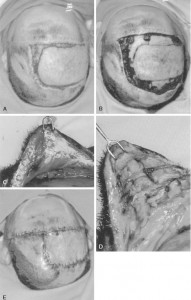
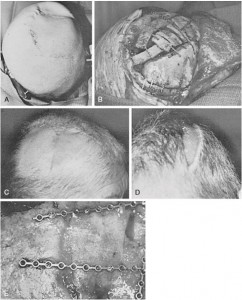
Scalp reconstruction and split cranial bone cranioplasty after removal of a recurrent meningioma that had been previously irradiated. A bipedicle flap was advanced to replace the unstable skin. The secondary defect was resurfaced with a skin graft. A, Intraoperative appearance from behind and above. Note that positioning allows access to and exposure of as much skull as possible. B, Reconstruction of a right temporoparietal defect from behind, with split cranial bone stabilized with microplates and screws. C, Postoperative appearance from the right side of the reconstruction site. D, Frontal postoperative appearance of a skin-grafted secondary defect after rotation of the bipedicle flap. E, Appearance of skull reconstruction at the time of reoperation, 2 years later.
CASE REPORT 1: SCALP AND SKULL RECONSTRUCTION WITH A LOCAL FLAP AND CRANIAL BONE GRAFTS
A 65-year-old woman was referred for treatment of a recurrent meningioma arising in the right parietal area. This patient had undergone high-dose radiation treatment for the lesion as well as extirpative surgery. Complications led to loss of the bone flap in this area. On preoperative examination, the patient was found to have extremely atrophic skin in the area of her previous surgery and radiation and a large parietal skull defect beneath it.
The patient’s previous craniotomy scars were reopened, the recurrent tumor was removed, and a dural repair was performed. A separate bone flap in the occipital area was harvested, and the inner table removed using the Midas Rex drill. The outer table of the occipital bone was returned to its anatomic position. The right parietal defect was repaired with the bone harvested from this flap, and ï¬xation was provided with microplates and screws. At the time of closure, the unstable skin was excised and replaced with a sagittally oriented bipedicle flap, which was harvested in a supraperiosteal plane. The resultant defect located in the left parietal area was skin grafted with a meshed, split-thickness skin graft that was harvested from the left lateral thigh. This graft was meshed 1 to 1.5 times and placed nonexpanded on the secondary skull defect.
The patient’s postoperative course was unremarkable. Two years later, another meningioma was found in an adjacent area. During this surgery the anterior aspect of the previous operative site was exposed to reveal complete healing of the previous reconstruction of the skull vault.
Free FlapsÂ
Closure of near total scalp defects or even smaller scalp defects in the setting of previous scalp radiation requires the use of free tissue transfer. A free flap is one that is completely detached from the donor area and moved to another site on the body. After this movement, circulation to the flap is restored by the microsurgical anastomosis of flap and available recipient site vasculature. The superï¬cial temporal artery or other branches of the external carotid system in the neck most often provide donor site vasculature for free tissue transfers used to reconstruct the scalp.
FIGURE 139-7
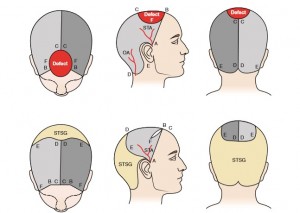
Upper : Two large parieto-occipital fl aps based on the superï¬cial temporal arteries (STA) are designed and rotated anteriorly. The defect is colored in red. The defect sagittal diameter (B and C) must be equal or less than the sagittal length of both flaps (C and D). The defect coronal diameter (F ) must be equal or less than the sum of the posterior diameter of the flap (D and E). Lower : Postoperative views of the flap. The occipital (OA) and posterior auricular arteries are ligated. The secondary defect is covered with split-thickness skin graft (STSG) . (Figure modiï¬ed from Papadopoulos O, Karypidis D, Moustaki M, et al. Double scalping fl ap: a versatile technique in scalp reconstruction. J Craniofac Surg. 2009;20:1484-1491.)
Since Miller ï¬rst reported use of a microsurgical technique in the scalp in 1976, many different free flaps have been used to cover large scalp defects. The latissimus dorsi muscle provides the best coverage because of its large surface area and long vascular pedicle 23 ( Fig. 139-8 ; see case report 2). Other free flaps used to cover the scalp include anterolateral thigh, radial forearm, parascapular, omentum, rectus abdominus myocutaneous flap, and even scalp from an identical twin. 24-28
The addition of free flaps to the reconstructive surgeon’s armamentarium makes almost any size or type of wound reconstructable 29 in a single-stage procedure. However, they have donor site morbidity and they are time consuming. Therefore, they are reserved for situations when local flaps or skin graft is not an appropriate option.
CASE REPORT 2: SCALP RECONSTRUCTION WITH A FREE TISSUE TRANSFERÂ A
58-year-old previously healthy woman was referred with a 6-month history of a rapidly growing scalp tumor. The preoperative evaluation revealed no evidence of tumor spread beyond the scalp. A biopsy revealed that the tumor was an angiosarcoma.
The entire tumor, along with a 2-cm margin of grossly normal-appearing skin, was removed down to the outer table of the skull. In addition, a left-sided parotidectomy was performed. The defect was reconstructed with a latissimus dorsi muscle – free tissue transfer. The muscle was then covered with a split-thickness skin graft that was harvested from the left thigh.
The patient’s postoperative course was unremarkable. Chemotherapy was administered postoperatively. The patient died of metastatic disease approximately 2.5 years after surgery.
Tissue ExpansionÂ
Tissue expansion makes use of the long-observed principle that skin expands to accommodate itself to gradual stretching. In 1978, Radovan was the ï¬rst to report successful clinical applications of this observation and to demonstrate prototypes of the expanders in clinical use today. 9-11 The expanders that are currently in use are silicone bags with self-sealing valves placed beneath the areas to be expanded. 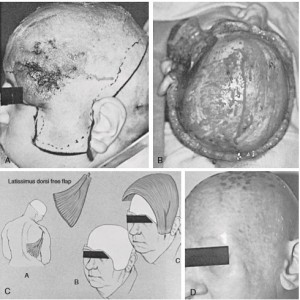
FIGURE 139-8
A, Preoperative lateral view of a scalp tumor. B, Intraoperative view of the defect from above and behind. C, Diagrammatic representation of the procedure. D, Postoperative result.
Tissue expansion has become a preferred technique of scalp reconstruction because it provides hair-bearing skin for reconstruction. Up to 50% of the scalp can be reconstructed with tissue expanders 30 without a clinically noticeable change in the density of hair follicles in the expanded skin. Unlike many rotation flaps, the resultant advancement flap created by the expansion process does not appreciably change the orientation of the hair follicles. 20
Tissue expanders are available in different sizes and shapes. In general, rectangular expanders (38%) give the most in tissue gains when compared to crescentic (32%) and round (25%) bases. 31 Tissue expanders are placed adjacent to the defect in a subgaleal pocket. Saline may be injected percutaneously through a one-way valve into the expander once or twice per week to increase the volume. The expansion of tissue expanders should proceed until the expanded scalp is 20% bigger than the size of the wound to account for tissue recoil during flap advancement. 32 The duration required to inflate the expander varies with each clinical situation, but it is usually between 4 and 8 weeks.
At a second operation, the expander is removed, and the resultant scalp fl ap is used to close the defect. Disadvantages of tissue expansion include the need for two or more operations, the interim deformity, and usually the fact that the expander cannot be placed in the acute setting. Tissue expanders are most often placed after the wound has been closed temporarily by simpler but less deï¬nitive techniques ( Fig. 139-9 ; see case report 3).
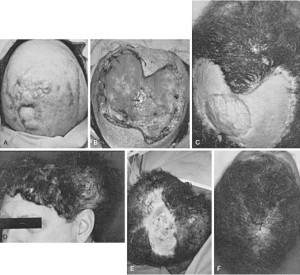
CASE REPORT 3: SCALP RECONSTRUCTION WITH SKIN GRAFT AND TISSUE EXPANSION
A 47-year-old man was referred with a massive dermatoï¬ brosarcoma protuberans carcinoma of the scalp, which had been treated 8 years previously with a topical solution. A preoperative evaluation revealed clinical and CT evidence that the tumor focally involved the outer table of the skull.
The lesion was excised together with a 2-cm margin of grossly normal-appearing tissue. Removal resulted in a defect down to the periosteum of 17 × 12 cm. Where the tumor was adjacent to the outer table, the outer table was excised to an area 8 cm 2. The wound was immediately closed by the placement of a meshed, split-thickness skin graft of approximately 0.014-inch thickness on the patient’s periosteum and drilled-down outer table. This recipient site was dressed with Adaptic and saline-soaked gauze, which was immobilized by sewing the dressing to the intact scalp.
FIGURE 139-9
This patient underwent radical excision of a scalp cancer and staged reconstruction using tissue expansion. A, Preoperative appearance of a scalp tumor. B, An intraoperative view of the resultant scalp defect. C, Postoperative appearance after placement of meshed split-thickness skin graft. The central area is where the outer table was removed and the split graft was placed on the diploë. D, Appearance with tissue expander in place. Scalp expansion and advancement were performed twice to close the defect. E, Intraoperative appearance during removal of a second set of expanders. F, Postoperative appearance after removal of the second set of expanders, flap advancement, and closure.  Â
The dressing was removed after 7 days, at which time the graft had almost completely taken. The area was observed for a recurrence for 1 year, but none occurred. During this time, small areas of skin graft breakdown occurred; these areas were managed with local dressing care. Approximately 1.5 years after radical removal of the tumor, two large tissue expanders were placed in the hair-bearing skin in the occipital and temporal areas. These expanders were gradually ï¬lled over the next 10 weeks, after which the expanders were removed and the resultant scalp flap was advanced. This action resulted in a remaining defect of approximately 8 × 10 cm. Two more tissue expanders were placed, and the expansion process was repeated. At the time of removal of the second set of expanders, the entire area of skin-grafted skull was resurfaced with hair-bearing skin.
Conclusions
Reconstruction of the scalp requires careful clinical examination to provide optimal deï¬nition of the real or anticipated defect and the status of the adjacent scalp. The size and location of a soft-tissue defect will determine the complexity of procedures required for reconstruction. Full thickness skin coverage for the skull can usually be provided either by galeal scoring and direct advancement or by the creation of a local flap. As noted earlier, galeal scoring and advancement of wound edges usually close defects up to 3 or 4 cm  in diameter. Defects larger than this can be closed by creating a rotation or bipedicle flap and by closing the secondary defect with a split-thickness skin graft on the skull periosteum. Large and complex defects usually require free tissue transfers. Tissue expanders are most often placed in the scalp secondarily to reconstruct alopecia defects with neighboring hair-bearing tissue.
KEY REFERENCES
Argenta L C, Morykwas M J. Vacuum-assisted closure: a new method for wound control and treatment. Clinical experience.  Ann Plast Surg.1997; 38:563 – 577.
Arnold P G, Rangarathnam C S. Multiple-flap scalp reconstruction: orticochea revisited. Plast Reconstr Surg. 1982;69:605 – 613.
Borah G L, Hidalgo D A, Wey P D. Reconstruction of extensive scalp defects with rectus free flaps. Ann Plast Surg. 1995;34:281 - 285.
Buncke H J,  Hoffman  W Y, Alpert B S, et al. Microvascular transplant of two free scalp flaps between identical twins. Plast Reconstr Surg. 1982;70:605 - 609.
Chicarilli Z N, Ariyan  S,  Cuono C B. Single-stage repair of complex scalp and cranial defects with the free radial forearm flap. Plast Reconstr Surg.1986;77:577- 585.
Elliott L F, Jurkiewicz T. Scalp and calvarium. In: Jurkewicz T, Mathes  S, Ariyan S, eds. Plastic Surgery: principles and Practice. St. Louis: Mosby; 1990:419 - 440.
Gordon L, Buncke H J, Alpert B S. Free latissimus dorsi muscle flap with split-thickness skin graft cover: a report of 16 cases. Plast Reconstr Surg. 1982;70:173 - 178.
Ikuta  Y. Microvascular free transfer of omentum. In: Vasconez LO , S trauch B, eds.
Grabb’s Encyclopedia of Flaps. Philadelphia: Lippincott-Raven; 1998:42 - 44.
Koss N, Robson M C, Krizek  T J.Scalping injury. Plast Reconstr Surg. 1975;55:439 - 444.
Leedy  J E , Janis J E , Rohrich R J. Reconstruction of acquired scalp defects: an algorithmic approach. Plast Reconstr Surg. 2005;116: 54e - 72e.
Lutz B S. Aesthetic and functional advantages of the anterolateral thigh fl ap in reconstruction of tumor-related scalp defects. Microsurgery. 2002;22:258 - 264     .
Manders  E K, Graham III W P, Schenden M J , Davis TS. Skin expansion to eliminate large scalp defects. Ann Plast Surg. 1984; 12:305 - 312.
Marathe U S, Sniezek J C. Use of Vacuum-Assisted Closure Device in Enhancing Closure of a Massive Skull Defect. Paper presented at Weste rn Section Meeting of the Triological Society, January 31, 2003.
Molnar J A, DeFranzo A J, Marks MW. Single-stage approach to skin grafting the exposed skull. Plast Reconstr Surg. 2000;105:174 - 177.
Nair S, Giannakoupoulous  G. Surgical management of radiated scalp in patients with recurrent glioma.       Neurosurgery. 1994; 34( 1 ): 103 - 107.
Nordstrom R .  “Stretch-back†in scalp reductions for male pattern baldness. Plast Reconstr Surg.  1984;73:422- 426.
Nordstrom R E A, Devine  J W . Scalp stretching with a tissue expander for closure of scalp defects. Plast Reconstr Surg. 1985;75:578 - 581.
Orticochea M. New three-flap scalp reconstruction technique. Br J Plast Surg. 1971;24:184 - 188.
Papadopoulos O, Karypidis D, Moustaki  M, et al. Double scalping flap: a versatile technique in scalp reconstruction. J Craniofac Surg. 2009; 20:1484 - 1491.
Radovan C . Tissue expansion in soft-tissue reconstruction. Plast Reconstr Surg. 1984;74:482 -Â 492.
Schmidt D, Adelmann G. The course of the occipital artery—an anatomical investigation for biopsy in suspected vasculitis. Eur J Med Res. 2001;6:235 - 241.
Schwenn O K, Wustenberg E G, Konerding M A , Hattenbach  L O. Experimental percutaneous cannulation of the supraorbital arteries: implication for future therapy. Invest Ophthalmol Vis Sci.  2005;46: 1557- 1 560.
Urken M K, Catanlano P J, Sen C. Free tissue transfer for skull base reconstruction: an analysis of complications and a classiï¬cation system for deï¬ning skull base defects. Arch Otolaryngol Head Neck Surg. 1993; 119:1318 - 1325.
Van Rappard J H, Molenaar  J, van Doorn K, et al. Surface-area increase in tissue expansion. Plast Reconstr Surg.1988;82:833 - 839.
Zide B. Surgical Anatomy around the Orbit: the System of Zones. Philadelphia: Lippincott; 2005Â Â Â Â Â Â Â Â .
Numbered references appear on Expert Consult.

Leave Comments
You must be logged in to post a comment.